Class 4 Medicines Defect Information: Keppra 500mg film-coated tablets (Doncaster Pharma Limited)
Drug alert number: EL (24)A/15
Date issued: 15 May 2024
The Medicines and Health products Regulatory Agency (MHRA) has issued a class 4 medicines defect information notice for: Keppra 500mg film-coated tablets (Doncaster Pharma Limited)
Company name: Doncaster Pharma Limited
Product name: Keppra 500mg film-coated tablets, PL 56830/0005
SNOMED Code: N/A
Batch Number
Expiry date
Pack size
First distributed
341173/BA
07/2024
60 tablets
21/08/2023
366686/BA
05/2025
60 tablets
21/08/2023
359701/BA
06/2025
60 tablets
21/08/2023
366621/BA
07/2025
60 tablets
21/08/2023
366635/BA
07/2025
60 tablets
21/09/2023
366635/BB
07/2025
60 tablets
21/09/2023
366101/BA
07/2025
60 tablets
06/11/2023
366635/BC
07/2025
60 tablets
06/11/2023
369420/BA
08/2025
60 tablets
01/12/2023
369421/BA
08/2025
60 tablets
01/12/2023
369421/BB
08/2025
60 tablets
25/01/2024
371089/BA
07/2025
60 tablets
01/02/2024
367482/BA
07/2025
60 tablets
01/02/2024
369420/BB
08/2025
60 tablets
01/02/2024
380087/BA
02/2026
60 tablets
05/02/2024
357677/BA
01/2025
60 tablets
12/03/2024
346651/BA
09/2024
60 tablets
02/04/2024
366635/BD
07/2025
60 tablets
08/04/2024
371089/BB
07/2025
60 tablets
11/04/2024
371016/BA
08/2025
60 tablets
11/04/2024
369420/BC
08/2025
60 tablets
11/04/2024
379799/BA
12/2025
60 tablets
11/04/2024
380087/BB
02/2026
60 tablets
11/04/2024
381719/BA
02/2026
60 tablets
11/04/2024
369421/BC
08/2025
60 tablets
23/04/2024
380089/BB
02/2026
60 tablets
23/04/2024
371016/BB
08/2025
60 tablets
24/04/2024
380089/BA
02/2026
60 tablets
24/04/2024
Active Pharmaceutical Ingredient: Levetiracetam
Brief description of the problem
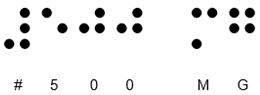
The MA holder, Doncaster Pharma Limited have identified an error relating to the Braille printed on the cartons on above parallel imported packs which have been repackaged by BModesto B.V. Approximately 70% of the packs across the listed batches have been repackaged with the Braille message on the Keppra 500mg film-coated tablets incorrectly stating strength as 1000mg.
Correct Braille
The correct Braille message should read:
Incorrect Braille
The incorrect Braille message reads as:
Advice for healthcare professionals
There is no issue with the quality of the tablets, therefore, the affected batches are not being recalled. We would advise healthcare professionals to check, before dispensing the medication, whether patients or carers handling or taking the medication will be relying on the Braille. Where this is the case, alternative batches should be dispensed to avoid confusion and subsequent underdosing.
View full alert here.
Advice for patients
Specific batches of Keppra 500mg film-coated tablets have an incorrect strength printed in Braille on the outer pack, it reads 1000mg instead of 500mg. The pack contains 500mg tablets as prescribed and quality of the medicine itself is not affected by this defect. Patients are reminded to take the tablets as per the instructions from your healthcare professional and those found on the dispensing label. If there are any concerns, consult with your healthcare professional. Never stop taking medicines such as Keppra without medical advice. Suddenly stopping an epilepsy medicine may cause your seizures to start again or happen more often or last longer than before.
Patients who experience adverse reactions or have any questions about the medication, should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Further Information
For medical information and stock control enquiries please contact Doncaster Pharma on telephone: 01302 365 000.
Email quality.enquiries@doncasterpharma.co.uk or commercial@doncasterpharma.co.uk
The post MHRA Class 4 Medicines Notification: Keppra 500mg film-coated tablets (Doncaster Pharma Limited) appeared first on Community Pharmacy England.
